25 years of helping to get the best care to people fast

Introduction
For 25 years, the National Institute for Health and Care Excellence (NICE) has acted as a beacon in health and care. It has helped practitioners and commissioners get the best care to people fast, while ensuring value for the taxpayer.
As NICE celebrates its 25th anniversary, we look back on its journey from its humble beginnings in 1999. We explore NICE's role and the wide-ranging impact it has had on health and care. And we provide a summary of the organisational changes NICE is making to ensure it continues to add value to an evolving health and care system.
A message from Dr Sam Roberts, chief executive of NICE, on our 25th anniversary



From small seeds to a global reputation
NICE was launched in April 1999 by the then Health Secretary, Frank Dobson MP. Sir Michael Rawlins was appointed as NICE's first chair and Sir Andrew Dillon as the organisation's first chief executive.
NICE's role was clear from the outset: to evaluate the clinical effectiveness and cost effectiveness of new medicines, technologies and interventions.
Prior to NICE, decisions about which drugs to fund had usually been taken at a local level. This led to concerns that people in some areas of the country could access treatments that people elsewhere could not. The situation was described by some as ‘postcode prescribing’.
There were consequently calls for a national, consistent approach in deciding what medicines and treatments the NHS should provide universally. The basis for this should be what worked, and what was cost-effective.
When NICE was established, the directions from the then Secretary of State for our role and purpose were clear: NICE should take into account the costs and benefits of treatments and care in guidance recommendations. And it should encourage the effective use of resources.
We are committed to providing the best value for taxpayers' money and the most effective, fair and sustainable use of finite resources.
In 2012, our role as a non-departmental body was re-established in the Health and Social Care Act 2012.
Together, these constitutional requirements shape our mission: to help practitioners and commissioners get the best care to people fast, while ensuring value for the taxpayer.
A reputation based on independence, rigour, transparency
Our reputation is based on guiding principles we adhere to, which ensure we’re recommending the best treatments and reducing variations in practice.
Key to these principles are the independence, transparency and rigour with which we develop our guidance.
We use independent advisory committees to consider the evidence. This is to ensure that our recommendations are unbiased and objective, and that the evidence is interpreted in a way that is relevant to health and social care delivery in England.
We are transparent in our methods and processes. Our decisions are made publicly, and the methods and process that define our approach are regularly reviewed.
And we are rigorous in our approach. Our guidance is underpinned by evidence that is relevant, reliable and robust.
How we measure the effectiveness of new treatments?
Our process for judging the cost effectiveness of treatments and interventions has adapted over time.
But the way we measure the benefits of new treatments has remained consistent since we began.
We evaluate new medicines through the quality-adjusted life year (QALY) to measure their benefits.
QALYs are estimates that are calculated from a range of sources including questionnaires that ask about factors that impact on a person’s health-related quality of life.
We use the QALY to assess value for money – by assessing how much the treatment costs and how much benefit it produces compared with the next best available alternative. This is expressed as the ‘cost (in £) per quality-adjusted life year (QALY) gained’.

An organisation that has grown from small seeds
An organisation that has grown from small seeds

Sir Andrew Dillon CBE, the first chief executive of NICE
Sir Andrew Dillon CBE, the first chief executive of NICE

Sir Michael Rawlins, the first chair of NICE
Sir Michael Rawlins, the first chair of NICE

Helping practitioners and commissioners get the best treatments to people fast, while providing value to the taxpayer
Helping practitioners and commissioners get the best treatments to people fast, while providing value to the taxpayer

Our advisory committees are made up of independent members
Our advisory committees are made up of independent members

We are committed to providing he best value for taxpayer’s money and the most effective, fair and sustainable use of finite resources
We are committed to providing he best value for taxpayer’s money and the most effective, fair and sustainable use of finite resources

Celebrating the history of NICE




1999 to 2009 – the first 10 years
Making independent, rigorous and difficult decisions
Within months of being set up, we started work on our first drug appraisal. The appraisal was noteworthy as it immediately challenged us to put our principles into practice.
Chief medical officer for England, Sir Chris Whitty, on NICE at 25
Our guidance said that Relenza, an antiviral treatment for flu was not cost-effective and should not be provided on the NHS. This was because there was insufficient evidence to show Relenza reduced the severity of the illness for those most at risk: the elderly and people with asthma.
The decision set the tone for NICE as an organisation that issues rigorous, but fair challenges to the pharmaceutical industry.
Not long after, in 2000, we produced our first technology appraisal which recommended healthy wisdom teeth should not be removed as a precaution. The estimated cost savings to the NHS of this were £5 million.
First independent committees set up, clinical guidelines launched
In 2001, we started developing clinical guidelines, which remain a core part of our activity.
NICE guidelines are based on the best available evidence. They contain recommendations that are put together by experts, people using services, carers and the public.
Each guideline is developed according to a process that starts from the topic being chosen and extends to any future guideline updates.
In 2002, we published our first clinical guideline on the topic of schizophrenia. The guideline had a significant effect, as prior to this, there was little in the way of national guidelines for mental health. It has been used in Australia and the state of California and translated and adopted in Spain and Italy.
Since then, we have produced a raft of guidance on mental health conditions ranging from depression and anxiety, to eating disorders and maternal mental health.
Dianne Davies, NICE expert patient representative on her views of NICE
Integrating the voice of the public into our work
In 2002, we established the Citizens Council.
The Council was made up of members of the public and provided us with a public perspective on ethical issues such as fairness, which could then be taken into account when producing our guidance. The work of the Citizens Council was ground-breaking and widely respected across the health and care system.
We have since introduced NICE Listens, which expands and updates the important work of the Citizens Council. NICE Listens builds on the Citizens Council’s success and aims to address the challenges of the fast-changing health and care landscape in which we operate.
Now, instead of a standing group of council members, we invite a new sample of the public to take part. The group is given time to learn and become familiar with the topic area, before discussing it in detail.
The findings from NICE Listens help make sure our policies on complex and controversial issues reflect the values of informed members of the public.
Expanding to provide advice on surgical procedures and public health
Our role expanded in October 2002 when the interventional procedures advisory committee held its first meeting, looking at what operations should be offered to patients. The first guidance we published as part of this programme was on uterine artery embolisation for fibroids.
Dr John Dean, Royal College of Physicians and East Lancashire Hospitals NHS Trust, on NICE at 25
In 2002, NICE took on an additional new role of producing public health guidelines. Our debut public health guideline was on the use of brief interventions to reduce smoking.
With this new responsibility, came a change of name. We became known the National Institute for Health and Clinical Excellence. Decisions on which drugs should be available on the NHS also became legally binding, meaning the NHS has to pay for them if NICE recommends them where conditions are met.
Professor Andrew Furber, NHS regional director of public health, north west England, on NICE at 25
Our portfolio of products expanded further with 2 pioneering programmes to assess new medical technologies, devices and diagnostics.
The programmes have helped the NHS to make consistent decisions about which new technologies have the most potential to transform patient care while providing the best value for money for the health system.
2009-2019: setting the foundations for the future
In 2010 the government established the Cancer Drugs Fund (CDF) as a source of funding for cancer drugs in England.
This meant that new cancer drugs can be made available to people much more quickly than before.
A new approach to the CDF began in 2016. The new approach provides:
- access to promising new treatments, via managed access arrangement, while further evidence is collected to address clinical uncertainty
- interim funding for all newly recommended cancer drugs, giving people access to these treatments many months earlier than before.
By April 2024, over 100,000 people had benefitted from early access to treatments through the CDF.
Health and Social Care Secretary Victoria Atkins said: “This is a huge milestone and demonstrates the success of the Cancer Drugs Fund in fast-tracking patient access to ground-breaking new cancer treatments while allowing us to gather further real-world evidence on their effectiveness.”
By March 2024, nearly 100,000 people had benefitted from early access to NICE-recommended treatments through the CDF.
A change in name, expanded remit for social care, first highly specialised technologies guidance published

Professor David Haslam, former chair of NICE
Professor David Haslam, former chair of NICE
In 2012, Professor David Haslam was appointed as NICE's chair, and in the following year, NICE was re-established in primary legislation by the Health and Social Care Act 2012. This wider remit was reflected in a further change to our name, and NICE became the National Institute for Health and Care Excellence.
After taking on the remit of producing evidence-based guidance and standards for the social care sector in April 2013, our first piece of guidance was published in March 2014. The guidance outlined how medicines in care homes should be managed.
We've recommended 25 treatments for ultra-rare diseases since 2015.
A year later, we published our first highly specialised technologies (HST) appraisal. Our HST guidance looks at drugs for very rare conditions. Since 2015, we have recommended 25 treatments for ultra-rare diseases.
We published important guidance on combating antimicrobial resistance in 2017. This was accompanied by a series of guidelines covering the management of common infections, including cough, tonsillitis, sinusitis and sore throats.
A new milestone was reached at the start of 2018 with the publication of our 500th technology appraisal. We expect this number to double as we publish our 1000th technology appraisal in 2024.
2019-2024: a strategy for the future, new methods and processes, learning from the pandemic
In 2020, Professor Gillian Leng became NICE's chief executive and Sharmila Nebhrajani OBE took on the role of chairman.

Professor Gillian Leng, former chief executive of NICE
Professor Gillian Leng, former chief executive of NICE

Sharmila Nebhrajani OBE, NICE's chairman
Sharmila Nebhrajani OBE, NICE's chairman
The change of leadership arose at a time when the organisation had to react quickly to the new and exceptional pressures arising from the COVID-19 pandemic.
Overnight, we adapted processes so that staff could work remotely, and we could continue business during lockdowns.
It was clear that the health and care system required NICE to provide support during a particularly difficult and uncertain period.
We collaborated with researchers to identify potential new drugs for COVID-19 and produced rapid guidelines for clinicians on topics relevant to the pandemic.
In February 2022, we launched our new combined methods and process manual and our topic selection manual for health technology evaluation.
The manual outlines changed processes for new evaluations to give people earlier access to innovative new treatments, by allowing greater flexibility over decisions about value for money, and consideration of a broader evidence base.
They result from one of the largest and most comprehensive reviews of our health technology evaluations ever undertaken.

NICE in 2024:
Timely, relevant, usable
and impactful guidance
Since NICE was established in 1999, we’ve delivered vast amounts of guidance; more than many other health technology assessment bodies across the globe.
But health and care has changed rapidly since we were created.
Digital health technologies, with the potential to transform healthcare, are constantly emerging. Evidence-based healthcare is evolving. The amount of health and care data has grown exponentially. And the health and care system is facing unprecedented workforce and capacity pressures.
So, we've been taking measures to adapt.
Our principles, and fundamental priorities remain the same. But given these new pressures, we're evolving to meet the changing needs of our users.
We'll maintain our independence, transparency and rigour. But we will now also focus on:
- timeliness
- relevance
- impact
- usability.
Our transformation plan builds on the 5-year strategy we set out in 2021. But it sharpens our focus to make sure we’re addressing current challenges.
This transformation will ensure we can meet the opportunities and challenges of the changing health and care landscape. It also enables us to maintain our role in helping you to deliver the most effective and affordable care.
Timeliness
Timeliness
Relevance
Relevance
Impact
Impact
Usability
Usability
Timeliness:
helping you get the best care to people fast




Over the years, our approaches have meant the UK remains competitive when compared with other countries in Europe in terms of access decisions.
For example, England is 6th out of 37 countries in the EU in terms of time in days from regulatory approval to reimbursed access (EFPIA WAIT report 2023). Furthermore, the timeliness of our appraisals increased by 17%.
However, there is more that we can do to improve and sustain these figures over the long term. And we know that people want and expect faster access to new treatments, even in cases where NICE needs more data to make a final decision.
So, we are carrying out a range of projects to help improve the timeliness of our guidance.
A streamlined approach for simpler, low-risk treatments
In 2022, we introduced a new proportionate approach to technology appraisals.
We simplified, removed, or reconfigured parts of the appraisals process. This means we applied light-touch, faster approaches to simpler, low-risk treatments allowing us to produce rapid guidance for some topics.
We’ve recommended 6 treatments in the first phase of this project, benefitting around 350,000 people.
This helped speed up our technology appraisals process by up to 20 weeks (45%) and increased our capacity for guidance on more complex treatments.
Our streamlined approach to appraisals sped the process by up to 20 weeks (45%), benefitting 350,000 people.
We’re now applying lessons learned from this approach more widely, to simplify and speed up processes in other areas of our work.
Guidance coinciding with MHRA approval
In 2023, we achieved a significant milestone in providing guidance on a new treatment at the same time as it received its licence from the Medicines and Healthcare products Regulatory Agency (MHRA).
We recommended glofitimab for people with advanced lymphoma. The treatment works by encouraging the healthy cells in the body that are responsible for the immune system to destroy the cancer cells.
Shortly after, we published guidance on a new 1-a-day pill as an option for treating sever ulcerative colitis. The guidance published on the same day that the treatment was granted a licence by the MHRA, in what was a first for NICE.
Increasing speed even further
In 2024, the Medicines and Healthcare products Regulatory Agency launched a new international recognition procedure.
Dr June Raine, chief executive of Medicines and Healthcare products Regulatory Agency, on NICE at 25
We are using the opportunity to examine ways we can speed up our appraisals processes even further.
We are developing ways of enhancing horizon scanning, and issuing guidance on evidence requirements.
We will also work with our regulatory and industry partners to identify opportunities and methods for making our processes even speedier and more efficient.
Relevance – focusing on what matters most




As a health technology assessment body, with 25 years of experience, we’ve produced guidance and advice on a number of conditions and diseases ranging across diverse health and care settings.
But it is important that our advice continues to be relevant to the evolving pressures of the health and care system.
So, we are making sure that we’re focusing on what matters most. We’ve carried out activity that tackles the most urgent issues in health and care – from addressing system pressures, to a new resource to help tackle health inequalities, to developing a new subscription model for the evaluation and purchase of antimicrobials.
Helping a health and care system under strain
We recognise that the health and care system is under exceptional pressure.
We have been carrying out a range of activity that can support practitioners and commissioners with improving productivity and recovering core services.
In 2023, we published guidance that recommends doctors offer a home test to people with signs or symptoms of colorectal cancer, to spare the need and reduce the waiting times for a colonoscopy.
This guidance is helping GPs to better identify and refer the right people for further testing quickly, freeing up waiting lists for those more likely to have a positive diagnosis. Around 100,000 fewer colonoscopies are expected to take place each year as a result.
We also published guidance recommending that radiotherapy for some breast cancer patients could be reduced without compromising safety.
First introduced during COVID, this approach is now enshrined in our guidance, with 10,000 appointments freed up for every 1,000 patients undergoing shorter treatment.
Helping the NHS improve productivity
In 2023, we launched a new web resource that matches relevant NICE guidance and recommendations to areas of improving productivity and recovering core services for the NHS.
Each piece of guidance or recommendation is highlighted with the related productivity opportunity. For example, it might help reduce the length of a hospital stay, reduce A&E attendance, improve the speed of rehabilitation, or speed up discharges.
The resource is primarily aimed at commissioners and integrated care boards and will be continually updated with latest relevant guidance supporting NHS priorities.
We will continually update the resource with relevant guidance to support NHS priorities.
Virtual wards
Virtual wards (also known as hospital at home) provide hospital level care and monitoring outside of a hospital setting.
The NHS has an ambition for virtual wards to expand the capacity of the acute care sector by managing people, who would otherwise be in hospital, remotely in their homes.
We recognise the potential benefits they have in releasing capacity. But we’re also aware of the challenges they may raise.
So in 2023, we launched a range of guidance and resources to help practitioners and commissioners implement this new model of care.
We've started by looking at the acute respiratory infection (ARI) pathway, aiming to help reduce pressure on the NHS over winter. We have since published a suite of guidance and resources to help manage ARI patients safely in their homes by implementing this new model of care.
Health inequalities
Reducing health inequalities is part of NICE’s DNA. It’s one of our core principles.
The pandemic exposed the impact that health inequalities have on our society. They are caused by the conditions in which we are born, live, work and grow. These conditions influence how we think, feel and act and can affect both our physical and mental health and wellbeing.
Reducing health inequalities part of NICE’s DNA. It’s one of our core principles.
We have developed a programme of work on health inequalities, which embed the consideration of health inequalities in the production of our guidance. It also ensures NICE focuses its efforts on supporting the health and care system to reduce health inequalities, where it will have the highest impact.
Our health inequalities web resource, first launched in 2022, pulls together all our guidance and resources on the topic. The resource maps all our guidance onto existing health inequalities frameworks such as Core20PLUS5, and is regularly updated to include new guidelines.
Antimicrobial stewardship
Resistance to antimicrobials is complex and increasing. There is a growing risk that infections may not be treatable in the future, combined with a lack of new antimicrobial medicines.
Over the years, we have developed a suite of guidance that encourages the safe and effective use of antibiotics.
We published milestone guidance in 2022, that recommends 2 new antimicrobial drugs. The guidance followed a new subscription-style payment, model, developed with NHS England, that has been designed to try and address the lack of new antimicrobials being developed and the growing threat posed by antimicrobial resistance.
By using subscription contracts instead of paying for each dose used, this new NHS payment model aims to incentivise antimicrobial companies to develop new products, while also upholding the important principles of antimicrobial stewardship. It is an approach that seeks to limit the growth of resistant infections caused by an overuse of antimicrobials.
Making an impact on the biggest areas in health and care
Since our inception in 1999, our guidance has had a wide-ranging impact in improving the lives of people.
This impact ranges from the reduction in the number of unnecessary surgery and invasive tests being carried out, to producing guidance on ground-breaking new treatments.
Helping practitioners and commissioners tackle cancer
Cancer is a condition that touches all our lives. It is among the long-term priorities for the NHS. And it is an area where NICE has contributed to through multiple pieces of guidance on effective treatments.
Since 2000, we have published more than 460 technology appraisals on cancer drugs, with 78% of our cancer appraisal recommendations being positive.
The guidance includes 42 treatments for lung cancer, 36 of which have been approved in the past 10 years.
Since 2000, we have published more than 460 technology appraisals on cancer drugs, with 78% of our cancer appraisal recommendations being positive.
As we mark our 25th year, we continue to develop guidance on the most innovative treatments. For example, in 2023 we recommended 2 new personalised immunotherapy treatments to treat aggressive forms of blood cancer, benefitting hundreds of people.
And hundreds of people can now access the first immunotherapy drug for an advanced form of cervical cancer.
Ground-breaking and transformative new treatments for diabetes
It is estimated that 5 million people will have diabetes in the UK by 2025 – a figure that has trebled since 1996.
Furthermore, around 10% of the entire NHS budget is spent on diabetes. So it is important that NICE focuses on this area, ensuring the best value for money technologies are available.
We have produced guidance on multiple treatments for diabetes over the years. This includes life-changing ‘artificial pancreas’ technology for people with type 1 diabetes that we recommended in 2023.
The innovative device continually monitors a person’s blood glucose, then automatically adjusts the amount of insulin given to them through a pump.
In April 2024, the NHS announced that it will be rolling out the device nationally, following the publication of NICE guidance.
Highlighting ineffective procedures
Our guidance highlights treatments or procedures that are ineffective or no longer effective, allowing us to spare thousands of hours of clinicians’ and peoples’ time.
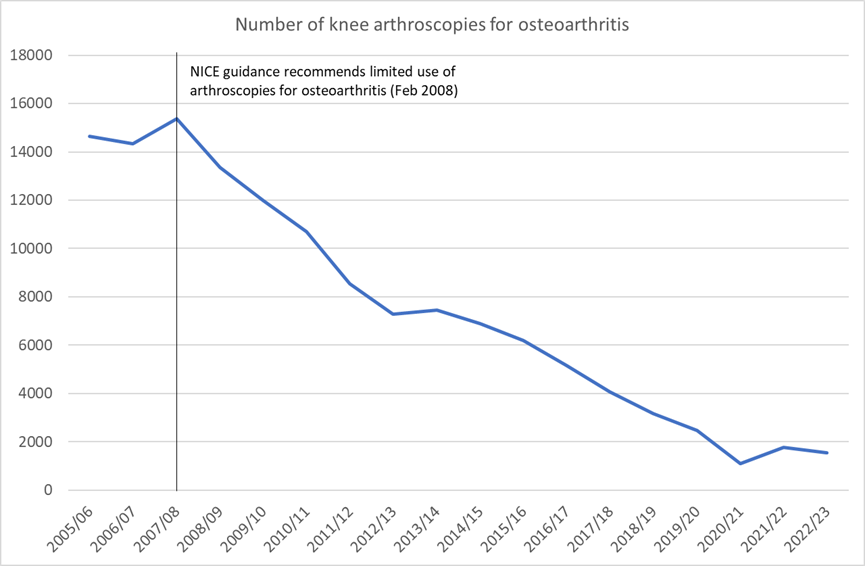
For example, there has been a 90% drop in the number of knee arthroscopies for osteoarthritis since our 2008 guidance recommended that they only be offered in very limited circumstances.


Making sure we provide useful and usable advice
Countless health and care practitioners have used our guidance over the years, benefitting the lives of thousands of people in doing so.
But we know that the needs of the health and care system are changing. So we’re adapting our processes, to ensure our guidance remains useful and usable.
A centralised approach to prioritising our guidance
We are developing a new approach for prioritising our guidance.
Through a centralised approach, we aim to produce guidance that is relevant, timely, accessible, and has demonstrable impact.
The approach has seen the creation of a central and integrated prioritisation board to decide on our priorities and coordinate delivery.
It also applies a common prioritisation framework, so that we consider all topics and products in a transparent and consistent way.
Including published technology appraisals in our guidelines
We’re bringing our guidance together by topic, so that it’s easier to access and clearer to understand.
This key project, which began in 2024, will allow us to include our published technology appraisals guidance in our guidelines. This means you’ll be able to access all our guidance on a topic in one place.
We expect the project will help increase the use of promising medicines and treatments in the NHS. This will lead to a more effective use of NHS resources, and better outcomes for people.
Advice on health technologies already in use
We are developing new ways of assessing technologies that are already in widespread use in the NHS, to support commissioning and procurement decisions.
This will help clinical practitioners, managers and commissioners to use NHS resources as effectively as possible, to ensure patient and system benefits are maximised, and to secure value for money.
Over time, technologies in use often undergo changes and adaptations. But there is little consistency in how these are valued and recognised. Through this project we will assess which technologies in a category represent value for money and whether price variations are justified by the incremental differences and changes.
Anticipating novel, innovative treatments for dementia
Recent estimates indicate there are over 850,000 people with dementia in the UK. The most common type of dementia is Alzheimer’s disease. Disease-modifying dementia treatments are currently being developed with the aim to alter the course of disease progression and reduce its substantial impact.
In 2023, we published a report that concluded that our methods and processes for evaluating the new class of Alzheimer’s drugs in the NHS are appropriate, while acknowledging that key issues need to be considered.
The report was produced by our Health Technology Assessment Innovation Laboratory (HTA Lab). The lab offers a ‘safe space’ for creating solutions in collaboration with system partners and stakeholders.
In addition to this work, the HTA Lab is currently developing proposals for the evaluation of artificial intelligence and genomics-based technologies that can be used in multiple disease areas.




An organisation fit for the future
Our renewed principles of timeliness, relevance, usability and impact, were developed from extensive conversations with our stakeholders.
They are based on the needs of our guidance users, and they will lay the foundations for a NICE of the future.
Through our recent work on streamlining our processes, updating our methods and processes, a new approach to prioritisation, and the integration of our products, we are developing an organisation fit for the next 25 years.
So, while the health and care system, and the environment we operate in, may change over time, we
know that a NICE of the future will be one where:

We focus on what matters most
We provide useful and usable advice

We constantly learn from data and implementation



For our users this will mean we are:

Focusing on what matters most
- We're developing more guidance that addresses major challenges to population health or the way the health and care system operates.
- We're aligning our methods between programmes to enable integrated guidance.
- We're building on our new NICE Advice service, which provides a single front door for early engagement with industry.
Providing useful and useable advice
- Faster guidance production across all NICE programmes.
- A new-look NICE website with easier navigation and clearer explanations of what we do.
- Guideline recommendations formatted in a way that makes it easier to read and easier to find what you’re looking for.

Constantly learning from data and implementation
- Developing a reproducible approach for automated monitoring of NICE guidance uptake, to enable implementation support and allow continuous evaluation.
Looking to the next 25 years and beyond…
The only constant in an ever-changing world is change itself.
Yet through an ever-changing health and care system, NICE has remained steadfast, as a beacon of stability, known for its transparency, rigour and independence.
As we celebrate our 25th anniversary, we can reflect on the numerous and significant ways in which NICE has had an impact on the health and care system. And we can expect more milestones to come, as we adapt to the requirements of evolving health and care system.
NICE looks forward to the future with a sense of purpose and excitement – keen to meet the opportunities and challenges ahead, and to continue to fulfil the expectations of practitioners, commissioners, and people of the future.
Stay informed about our work
Subscribe to our newsletters to keep up-to-date with important developments.
You’ll receive information on:
- our latest guidance
- implementation advice and case studies
- news, podcasts and blog posts
- upcoming consultations, meetings and events
- how you can engage with us.