NICE changes to provide faster, fairer access to new drugs and devices
We’re changing how we assess new drugs, devices, diagnostics and digital technologies to provide faster and fairer access in the NHS. The changes will also improve the way we work with patients, the NHS and the life sciences industry.
Here's what's changing and how to tell us what you think of our methods and process review.

This short animation summarises our proposed changes to health technology evaluation.
This short animation summarises our proposed changes to health technology evaluation.
What are the proposed changes?
As we see newer, more advanced technologies become available, we’re simplifying our approach and allowing more flexible decision-making, while continuing to be robust and responsive.
We heard from patients and charities that what matters most is helping people live well throughout life. We also found good evidence to support giving extra value to innovations that treat severe diseases at any stage, not only at the end of life. This means therapies for a wider range of physical and mental health conditions, such as severe epilepsy and multiple sclerosis, could be given additional value. We will also commission research to understand if we should go further in the additional value we are placing on medicines for severe disease as society’s views on this are important.
One panel of experts will decide what treatments, medical devices, interventional procedures and diagnostics we assess. This will allow for clearer, consistent and transparent topic selection decisions. We’ve also simplified criteria for very rare disease evaluations, including removing the criteria that the condition being treated should be chronic and severely disabling.


"We must balance ensuring the NHS gets the best value, delivering improved patient access and promoting innovation. These proposals mean we meet expectations to introduce effective products as soon as possible while maintaining a world-leading robust approach.”
Professor Gillian Leng CBE, NICE chief executive

What this means for patients, the NHS and life science industry



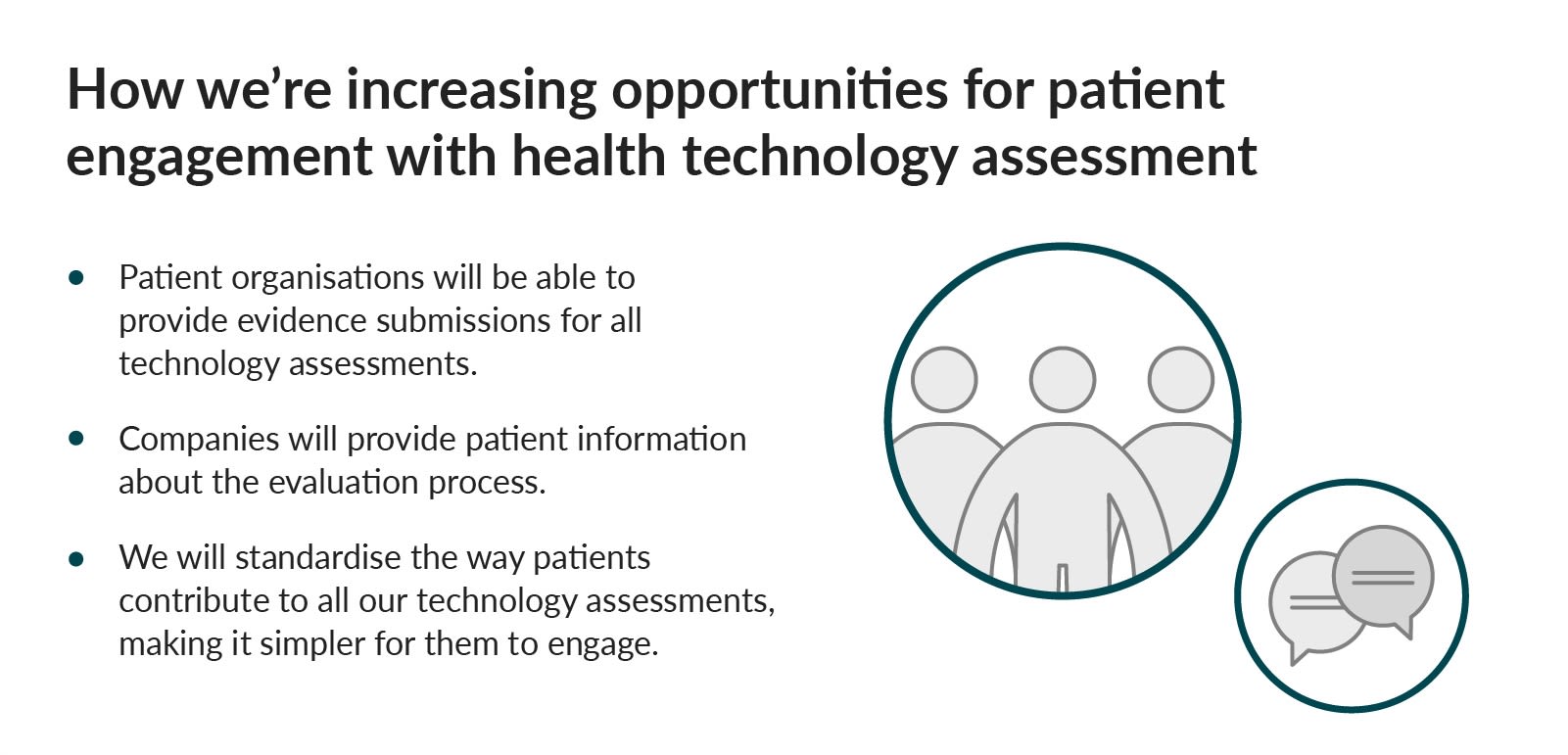
Patients in England will get faster access to a broader range of treatments and contributing to our evaluations will be easier.
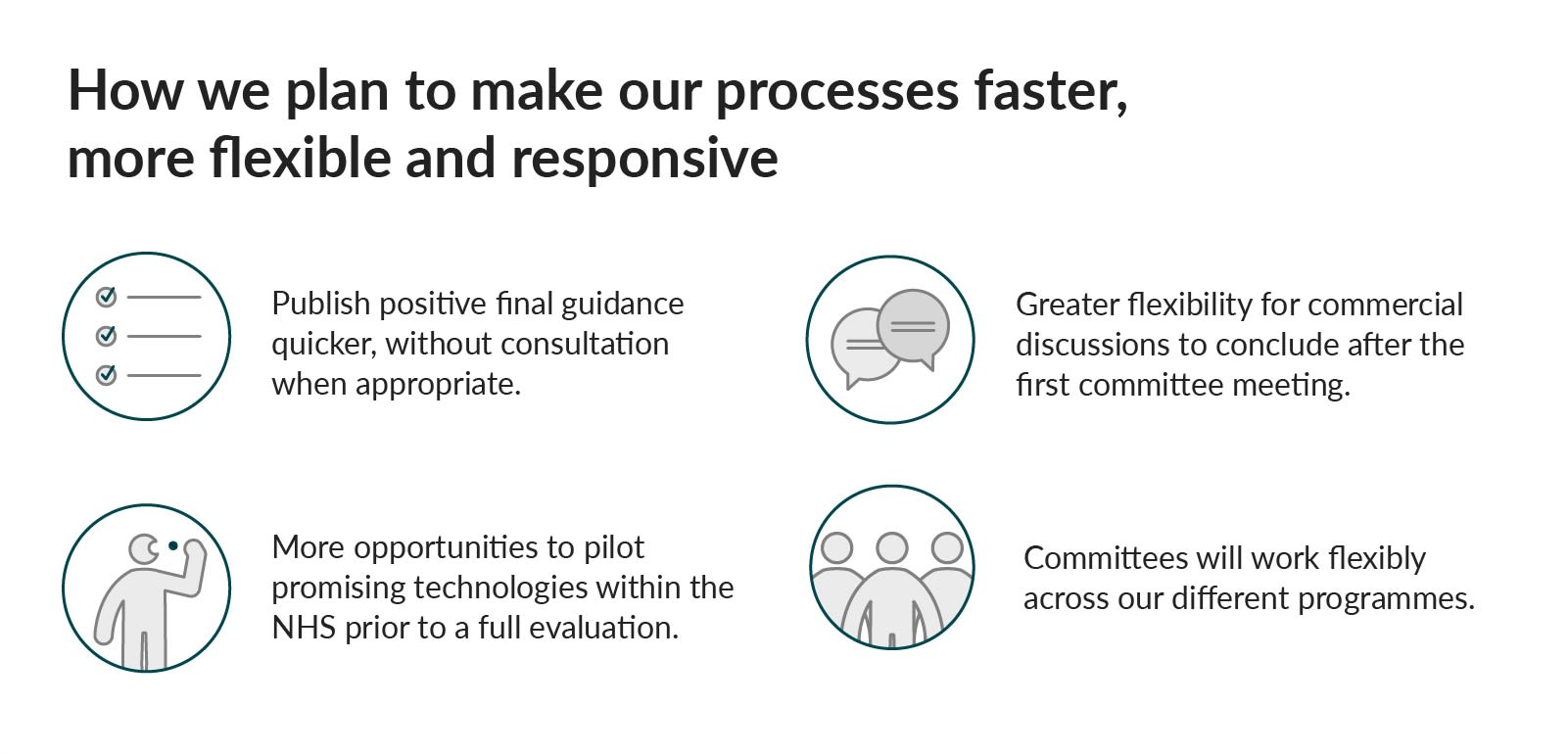
To speed up our processes for diagnostics and medical technologies, we’ll publish recommendations without consultation on all types of guidance, if the outcome is clear and unlikely to change. When we do need to consult there’ll be the option to shorten how long this should take.
We will expand managed access agreements – where companies offer new medicines at a reduced cost whilst evidence is gathered on their effectiveness – to promising but clinically uncertain medical technologies. This allows us to collect more data for an informed decision on routine access.
We’ll ask companies to provide lay evidence summaries to help patients understand our committee documents. We'll mainly keep all committee meetings virtual and patient organisations can provide written submissions.
The NHS will be the first to pilot the latest and most innovative treatments. We’ll support initiatives that let companies innovate and test their products in the UK ahead of launch. And we’ll aim to inform commercial discussions earlier.
Pharmaceutical and life science companies will benefit from flexible, responsive discussions to ensure innovations can be recommended without delay.
Our committees will have more flexibility where evidence generation is particularly difficult. For example with complex treatments, rare diseases and medicines for children. We’ll continue to develop methods to use new types of data sources including real-world evidence, expert testimony and qualitative surveys.

“This is a major milestone in an important review, and a significant opportunity to update the methods and processes NICE uses to support access to the life changing medicines of today and tomorrow. The ABPI and our members will be working hard over the coming months to help make sure the proposals meet the ambition of the UK’s Life Sciences Vision and deliver meaningful change for patients, their families, and the NHS.”

What stays the same

We’ll maintain our independence and continue to offer a world-leading approach with fair, transparent and robust evaluations. We remain committed to using evidence that is high quality, fit for purpose and appropriately critiqued.
We want everyone to have fair, equitable access to treatments that are clinically effective and value for money. And for us all to get the best value from our NHS.
Need more information?
We are holding a series of virtual events to help explain our proposals. Find out how to register.
We've also put together a useful summary document outlining our ambitions for the future of health technology evaluation.
